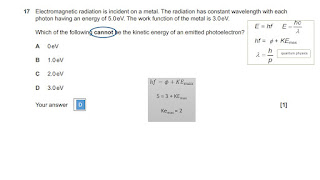
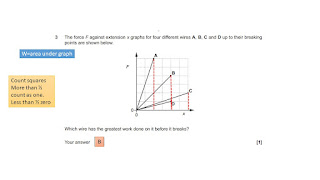
Prep
1. Calculate
the number of elementary entitles (atoms
or molecules) in 3.0 mol
of a substance.
2.
Suggest why one mole of silicon has a different
mass from one mole of aluminium.
3. Calculate
the number of moles there are in a substance containing:
2.0 × 1024
molecules ; 1.5 × 1017
atoms ; 2.0 × 1024
molecules.
4. The
molar mass of copper is 64 g mol–1 calculate the number of atoms in
copper
of mass 1.0 kg.
5. The
molar mass of uranium is 235 g mol–1. Calculate the mass of a single
atom of uranium.
6. The
density of lead is 11340 kgm−3. Each lead atom has a mass of 3.46 ×
10−25 kg. Calculate the number of moles of lead in a lead block with
a volume of 0.20 m3.
7. Calculate
the mass of 4.0 mol of helium-4 gas.
8. Calculate
the molar mass of methane (CH4).
9. Calculate the number of molecules in 50 g of
carbon dioxide.